Nociception (also nocioception or nociperception, from Latin nocere ‘to harm or hurt’) is the sensory nervous system’s response to certain harmful or potentially harmful stimuli. In nociception, intense chemical (e.g., chili powder in the eyes), mechanical (e.g., cutting, crushing), or thermal (heat and cold) stimulation of sensory nerve cells called nociceptors produces a signal that travels along a chain of nerve fibers via the spinal cord to the brain. Nociception triggers a variety of physiological and behavioral responses and usually results in a subjective experience of pain in sentient beings.
Detection of noxious stimuli
Potentially damaging mechanical, thermal, and chemical stimuli are detected by nerve endings called nociceptors, which are found in the skin, on internal surfaces such as the periosteum, joint surfaces, and in some internal organs. The concentration of nociceptors varies throughout the body; they are found in greater numbers in the skin than in deep internal surfaces. Some nociceptors are unspecialized free nerve endings that have their cell bodies outside the spinal column in the dorsal root ganglia. Nociceptors are categorized according to the axons which travel from the receptors to the spinal cord or brain.
Nociceptors have a certain threshold; that is, they require a minimum intensity of stimulation before they trigger a signal. Once this threshold is reached a signal is passed along the axon of the neuron into the spinal cord.
Nociceptive threshold testing deliberately applies a noxious stimulus to a human or animal subject in order to study pain. In animals, the technique is often used to study the efficacy of analgesic drugs and to establish dosing levels and period of effect. After establishing a baseline, the drug under test is given and the elevation in threshold recorded at specified time points. When the drug wears off, the threshold should return to the baseline (pre-treatment) value.
In some conditions, excitation of pain fibers becomes greater as the pain stimulus continues, leading to a condition called hyperalgesia.
Factors
The gate control theory of pain, proposed by Patrick David Wall and Ronald Melzack, postulates that nociception (pain) is “gated” by non-nociception stimuli such as vibration. Thus, rubbing a bumped knee seems to relieve pain by preventing its transmission to the brain. Pain is also “gated” by signals that descend from the brain to the spinal cord to suppress (and in other cases enhance) incoming nociception (pain) information.
Nociception can also cause generalized autonomic responses before or without reaching consciousness to cause pallor, diaphoresis, tachycardia, hypertension, lightheadedness, nausea and fainting.
System overview
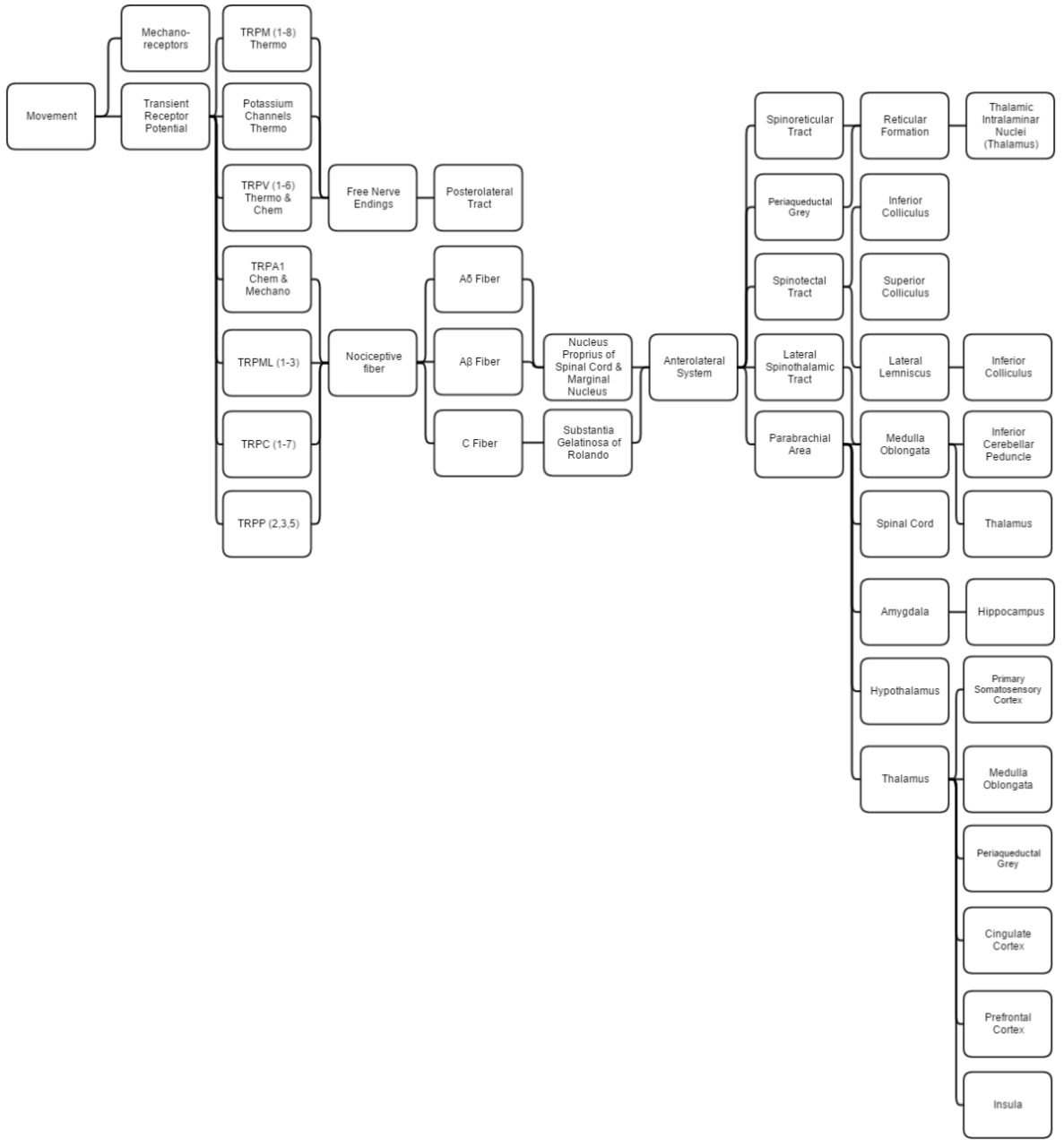
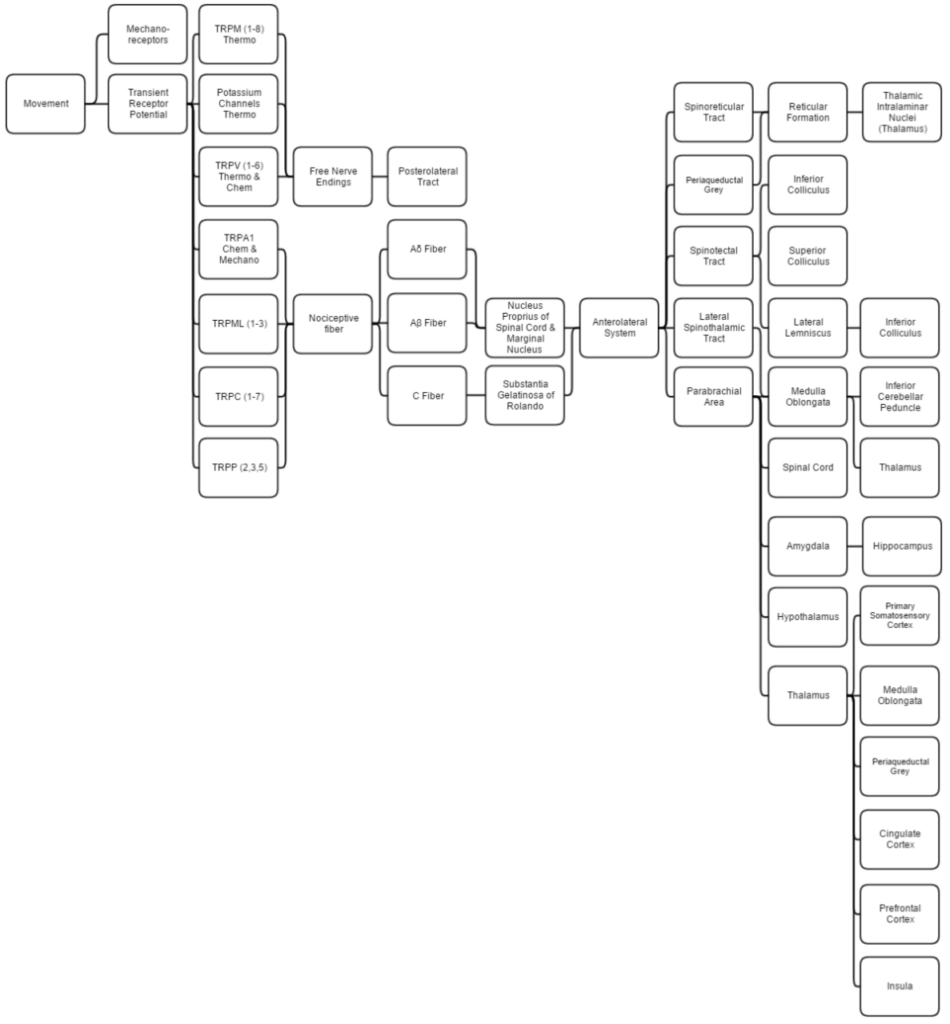
This diagram linearly (unless otherwise mentioned) tracks the projections of all known structures that allow for pain, proprioception, thermoception, and chemoception to their relevant endpoints in the human brain. Having trouble viewing? Click enlarge.
This overview discusses proprioception, thermoception, chemoception and nociception as they are all integrally connected.
Mechanical
Proprioception is determined by using standard mechanoreceptors (especially ruffini corpuscles (stretch) and transient receptor potential (TRP) channels). Proprioception is completely covered within the somatosensory system as the brain processes them together.
Thermoception refers to stimuli of moderate temperatures (~24-28C), as anything beyond that range is considered pain and moderated by nociceptors. TRP and potassium channels [TRPM (1-8), TRPV (1-6), TRAAK, and TREK] each respond to different temperatures (among other stimuli) which create action potentials in nerves which join the mechano (touch) system in the posterolateral tract. Thermoception, like proprioception, is then covered by the somatosensory system.
TRP channels that detect noxious stimuli (mechanical, thermal, and chemical pain) relay that info to nociceptors that generate an action potential. Mechanical TRP channels react to depression of their cells (like touch), thermal TRP change shape in different temperatures, and chemical TRP act like taste buds, signalling if their receptors bond to certain elements/chemicals.
Neural
Ab are the fastest, Aδ fibers are second, and C are slowest at 1/10th (2 m/s:20 m/s) Ao fiber’s speed. A fibers signal initial pain while C fibers signal deep, lasting pain. These fibers synapse on different spinal laminae (1-5).
- Laminae 3-5 make up nucleus proprius in spinal grey matter.
- Lamina 2 makes up substantia gelatinosa of Rolando, unmyelinated spinal grey matter. Substantia receives input from nucleus proprius and conveys intense, poorly localized pain.
- Lamina 1 primarily project to the parabrachial area and periaqueductal grey, which begins the suppression of pain via neural and hormonal inhibition. Lamina 1 receive input from thermoreceptors via the posterolateral tract. Marginal nucleus of the spinal cord are the only unsuppressible pain signals.
- The parabrachial area integrates taste and pain info then relays it. Parabrachial checks if the pain is being received in normal temperatures and if the gustatory system is active; if both are so the pain is assumed to be due to poison.
- Ao fibers synapse on laminae 1 and 5 while Ab synapses on 1, 3, 5, and C. C fibers exclusively synapse on lamina 2.
- The amygdala and hippocampus create and encode the memory and emotion due to pain stimuli.
- The hypothalamus signals for the release of hormones that make pain suppression more effective, some of these include sex hormones.
- Periaqueductal grey (with hypothalamic hormone aid) hormonally signals reticular formation’s raphe nuclei to produce serotonin that inhibits laminae pain nuclei.
- Lateral spinothalamic tract aids in localization of pain.
- Spinoreticular and spinotectal tracts are merely relay tracts to the thalamus that aid in the perception of pain and alertness towards it. Fibers cross over (left becomes right) via the spinal anterior white commissure.
- Lateral lemniscus is the first point of integration of sound and pain information.
- Inferior colliculus (IC) aids in sound orienting to pain stimuli.
- Superior colliculus receives IC’s input, integrates visual orienting info, and uses the balance topographical map to orient the body to the pain stimuli.
- Inferior cerebellar peduncle integrates proprioceptive info and outputs to the vestibulocerebellum. The peduncle is not part of the lateral-spinothalamic-tract-pathway; the medulla receives the info and passes it onto the peduncle from elsewhere (see somatosensory system).
- The thalamus is where pain is thought to be brought into perception; it also aids in pain suppression and modulation, acting like a bouncer allowing certain intensities through to the cerebrum and rejecting others.
- The somatosensory cortex decodes nociceptor info to determine the exact location of pain and is where proprioception is brought into consciousness; inferior cerebellar peduncle is all unconscious proprioception.
- Insula judges the intensity of the pain and provides the ability to imagine pain.
- Cingulate cortex is presumed to be the memory hub for pain.
In non-mammalian animals
Nociception has been documented in non-mammalian animals, including fish and a wide range of invertebrates, including leeches, nematode worms, sea slugs, and fruit flies. As in mammals, nociceptive neurons in these species are typically characterized by responding preferentially to high temperature (40° Celsius or more), low pH, capsaicin, and tissue damage.
History of term
The term “nociception” was coined by Charles Scott Sherrington to distinguish the physiological process (nervous activity) from pain (a subjective experience). It is derived from the Latin verb “nocēre”, which means “to harm”.