Scientific studies have found that numerous brain areas show altered activity in depressed patients. It has not been possible to determine a single cause of depression.
Monoamine hypothesis
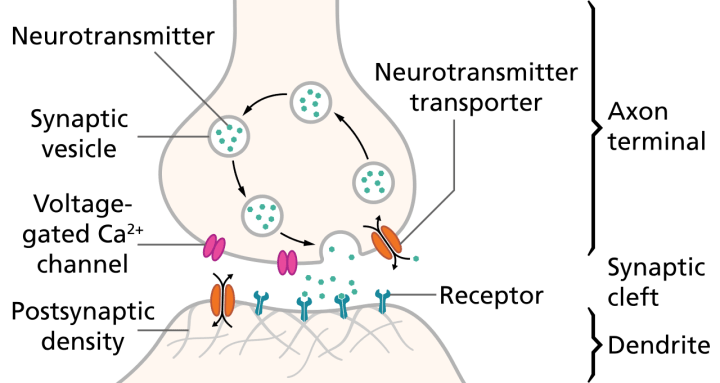
Illustration of the major elements in a prototypical synapse. Synapses are gaps between nerve cells. These cells convert their electrical impulses into bursts of chemical relayers, called neurotransmitters, which travel across the synapses to receptors on adjacent cells, triggering electrical impulses to travel down the latter cells.
Most antidepressants increase synaptic levels of the monoamine neurotransmitter serotonin. They may also enhance the levels of two other neurotransmitters, norepinephrine and dopamine. This observation gave rise to the monoamine hypothesis of depression. In its contemporary formulation, the monoamine hypothesis postulates that the deficit of certain neurotransmitters is responsible for the corresponding features of depression: “Norepinephrine may be related to alertness and energy as well as anxiety, attention, and interest in life; [lack of] serotonin to anxiety, obsessions, and compulsions; and dopamine to attention, motivation, pleasure, and reward, as well as interest in life.” The proponents of this hypothesis recommend choosing the antidepressant with the mechanism of action impacting the most prominent symptoms. The anxious and irritable patients should be treated with SSRIs or norepinephrine reuptake inhibitors, and the ones with the loss of energy and enjoyment of life—with norepinephrine and dopamine enhancing drugs.
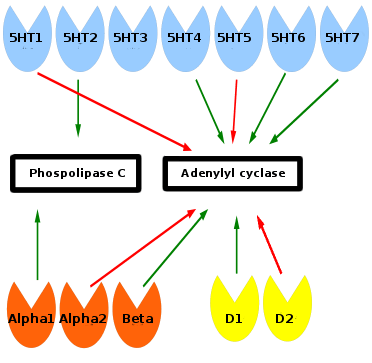
Monoamine receptors affect phospholipase C and adenylyl cyclase inside of the cell. Green arrows means stimulation and red arrows inhibition. Serotonin receptors are blue, norepinephrine orange, and dopamine yellow. Phospolipase C and adenylyl cyclase start a signaling cascade which turn on or off genes in the cell. The 5HT-3 receptor is associated with gastrointestinal adverse effects and has no relationship to the other monoamine receptors.
Consistent with the monoamine hypothesis, a longitudinal study uncovered a moderating effect of the serotonin transporter (5-HTT) gene on stressful life events in predicting depression. Specifically, depression seems especially likely to follow stressful life events, but even more so for people with one or two short alleles of the 5-HTT gene. Serotonin may help to regulate other neurotransmitter systems, and decreased serotonin activity may “permit” these systems to act in unusual and erratic ways. Facets of depression may be emergent properties of this dysregulation.
In the past two decades, research has uncovered multiple limitations of the monoamine hypothesis, and its inadequacy has been criticized within the psychiatric community. Intensive investigation has failed to find convincing evidence of a primary dysfunction of a specific monoamine system in patients with major depressive disorders. The antidepressants that do not act through the monoamine system, such as tianeptine and opipramol, have been known for a long time. Experiments with pharmacological agents that cause depletion of monoamines have shown that this depletion does not cause depression in healthy people nor does it worsen the symptoms in depressed patients. Already limited, the monoamine hypothesis has been further oversimplified when presented to the general public.
An offshoot of the monoamine hypothesis suggests that monoamine oxidase A (MAO-A), an enzyme which metabolizes monoamines, may be overly active in depressed people. This would, in turn, cause the lowered levels of monoamines. This hypothesis received support from a PET study, which found significantly elevated activity of MAO-A in the brain of some depressed people. In genetic studies, the alterations of MAO-A-related genes have not been consistently associated with depression. Contrary to the assumptions of the monoamine hypothesis, lowered but not heightened activity of MAO-A was associated with the depressive symptoms in youth. This association was observed only in maltreated youth, indicating that both biological (MAO genes) and psychological (maltreatment) factors are important in the development of depressive disorders. In addition, some evidence indicates that problems in information processing within neural networks, rather than changes in chemical balance, might underlie depression.
Circadian rhythm
Depression may be related to the same brain mechanisms that control the cycles of sleep and wakefulness.
Depression may be related to abnormalities in the circadian rhythm, or biological clock. For example, rapid eye movement (REM) sleep—the stage in which dreaming occurs—may be quick to arrive and intense in depressed people. REM sleep depends on decreased serotonin levels in the brain stem, and is impaired by compounds, such as antidepressants, that increase serotonergic tone in brain stem structures. Overall, the serotonergic system is least active during sleep and most active during wakefulness. Prolonged wakefulness due to sleep deprivation activates serotonergic neurons, leading to processes similar to the therapeutic effect of antidepressants, such as the selective serotonin reuptake inhibitors (SSRIs). Depressed individuals can exhibit a significant lift in mood after a night of sleep deprivation. SSRIs may directly depend on the increase of central serotonergic neurotransmission for their therapeutic effect, the same system that impacts cycles of sleep and wakefulness.
Research on the effects of light therapy on seasonal affective disorder suggests that light deprivation is related to decreased activity in the serotonergic system and to abnormalities in the sleep cycle, particularly insomnia. Exposure to light also targets the serotonergic system, providing more support for the important role this system may play in depression. Sleep deprivation and light therapy both target the same brain neurotransmitter system and brain areas as antidepressant drugs, and are now used clinically to treat depression. Light therapy, sleep deprivation and sleep time displacement (sleep phase advance therapy) are being used in combination quickly to interrupt a deep depression in hospitalized patients.
Abnormalities by brain region
Research on the brains of depressed patients usually shows disturbed pattern of interaction between multiple parts of the brain. Here are the areas that are most strongly affected:
Raphe nuclei
The raphe nuclei are a group of small nuclei in the upper brain stem, located directly at the midline of the brain. They are the sole source of serotonin in the brain. Despite their small size, they project very widely, and are involved in a very diverse set of functions. Most antidepressants are serotonergic. Serotonin system dysfunction cannot be the sole cause of depression, though: antidepressants usually bring serotonin levels up to normal very quickly, but it often takes at least two to four weeks before mood improves significantly. The 5-HTT gene regulates a chemical called serotonin. Serotonin works as the neurotransmitter and helps with the modulation of things such as anxiety, anger, appetite, sexuality, sleep, mood, and several other things. People with depression often have impaired 5-HTT genes. There are two forms of the 5-HTT gene and everyone has two 5-HTT genes. (Levinson) There is a long form of 5-HTT and a short form of 5-HTT. Research shows that people with both 5-HTT genes being the long form are less likely to become depressed while people with one short and one long or two short forms are more likely to develop depression. Research is still being conducted to find more information. The functions of serotonin are difficult to describe in a simple way. In some circumstances serotonin seems to act as a signal of “repletion” or “satisfaction”. Thus, satiation after eating, and orgasm following sex, both produce release of serotonin. In animals that have hierarchical social structures, dominant individuals show higher levels of serotonin metabolites than lower-status individuals. In the brain, serotonin exerts a suppressive effect on both the reward system and punishment system, and therefore is likely to reduce the intensity of motivation whether aversive or appetitive. (One of the most common but least-discussed side effects of antidepressants is to reduce sex drive).
Suprachiasmatic nucleus (SCN)
The suprachiasmatic nucleus (SCN) is the control center for the body’s “biological clock”. It contains neurons whose activity waxes and wanes throughout the day. The output from the SCN controls the sleep/wake cycle as well as a number of other biological rhythms, such as fluctuations in body temperature. Disturbances of these cycles are a consistent symptom of depression, especially of the melancholic type. The “classic” pattern is for depressed people to have great difficulty falling asleep at night, and then to wake bolt upright at around 3 AM. The waking is usually preceded by a rise in body temperature, which in non-depressed people does not usually occur until several hours later. It is a common observation that antidepressants produce a return to normal sleep patterns before they produce an improvement in mood: if good sleep does not return, it is a strong sign that the treatment is not going to be effective. Conversely, disruptions to sleep are often the first indication of impending relapse.
There is a powerful interaction between the Raphe nuclei and the SCN. On one hand, the Raphe nuclei send a strong serotonergic projection to the SCN. In animal studies, this input has been shown to modulate the ability of light to reset the timing of the biological clock: the more serotonin, the stronger the effects of light. On the other hand, the biological clock exerts a strong influence on the Raphe nuclei: serotonin levels drop during sleep, and fall almost to nothing during REM (dreaming) sleep. It is worth noting that one of the characteristics of sleep in depressed people is that REM tends to appear very soon after sleep onset, whereas in non-depressed people it does not usually dominate sleep until the last hours, in the early morning. Antidepressants are powerful suppressors of REM.
Ventral tegmental area (VTA)
The ventral tegmentum (or ventral tegmental area) is a small area in the basal midbrain which is a critical part of the brain’s reward system. It sends projections to the nucleus accumbens that use the neurotransmitter dopamine. Addictive drugs universally increase the effects of dopamine in this system, whereas drugs that oppose dopamine produce anhedonia of the sort seen in depressed people. Dopamine-enhancers such as cocaine often relieve the lack-of-pleasure in depression, but the effects only last as long as a drug is present in the body: that is, they temporarily alleviate one of the main symptoms, but do not help to cure the disease.
Nucleus accumbens (NAc)
Long-term exposure to various unavoidable stress factors decreases dopamine release in the NAc shell, as it was shown in the forced swimming test, an animal model of depression.
Anterior cingulate cortex (ACC)
The anterior cingulate cortex is activated by negative experiences of many types, and consistently shows higher levels of activity in depressed people than in non-depressed people. The functions of the ACC are controversial, but one proposal is that it mediates the conscious experience of suffering. Several decades ago, trials were made of ablating parts of the ACC in an attempt to relieve intolerable pain in patients who were terminally ill. These patients reported that after the surgery, they could still perceive the physical sensations of pain, but they no longer found them distressing. (The effects of heroin and morphine are sometimes described in the same way.) Very recently, clinical experiments were made in using deep brain stimulation to temporarily inactivate the ACC in severely depressed patients. This was not effective in all cases, but in some patients very striking results were achieved, with a perceptible lifting of mood immediately apparent to the patient as soon as the stimulus was applied.
Subgenual cingulate
Recent studies have shown that Brodmann area 25, also known as Subgenual cingulate is metabolically overactive in treatment-resistant depression. This region is extremely rich in serotonin transporters and is considered as a governor for a vast network involving areas like hypothalamus and brain stem, which influences changes in appetite and sleep; the amygdala and insula, which affect the mood and anxiety; the hippocampus, which plays an important role in memory formation; and some parts of the frontal cortex responsible for self-esteem. Thus disturbances in this area or a smaller than normal size of this area contributes to depression. Deep Brain Stimulations of this area have been successful in reducing its elevated activity and thus curing depression in patients that could not be cured by anti-depressants.
Altered neuroplasticity
Recent studies have called attention to the role of altered neuroplasticity in depression. Pittenger and Duman have reviewed extensive research, demonstrating a convergence of three phenomena:
- Chronic stress reduces synaptic and dendritic plasticity
- Depressed subjects show evidence of impaired neuroplasticity (eg. shortening and reduced complexity of dendritic trees)
- Anti-depressant medications enhance neuroplasticity at both a molecular and dendritic level.
They conclude that disrupted neuroplasticity is an underlying feature of depression, and is reversed by antidepressants.
Hypothalamic-Pituitary-Adrenal (HPA) axis
The Hypothalamic-pituitary-adrenal axis is a chain of endocrine structures that are activated during the body’s response to stressors of various sorts. It often shows increased activation in depressed people, and drugs that reduce its activity are sometimes effective in reducing symptoms. The HPA influences many parts of the brain, including the Raphe nuclei.
Genetic factors
In 2003 Science published an influential study of Avshalom Caspi et al. who found that a gene-environment interaction (GxE) may explain why life stress is a predictor for depressive episodes in some individuals, but not in others, depending on an allelic variation of the serotonin-transporter-linked promoter region (5-HTTLPR). Soon after, the results were replicated by Kenneth Kendler’s group, raising hopes in the psychiatric genetics community. By 2007 there were 11 replications, 3 partial replication and 3 non-replications of this proposed GxE. However, two of the largest studies were negative. Two 2009 meta-analyses were also negative; one included 14 studies, the other just five, owing to different study selection criteria. A 2010 review of studies in this area found 17 replications, 8 partial replications (interaction only in females or only with one of several types of adversity), and 9 non-replications (no interaction or an interaction in the opposite direction). It also found a systematic relationship between the method used to assess environmental adversity and the results of the studies; all studies using objective indicators or structured interviews to assess stress replicated the gene–environment interaction fully or partially, whereas all non-replications relied on brief self-report measures of adversity. This review also found that both 2009 meta-analyses were significantly biased toward negative studies.
Other hypothesized genomic influences are BDNF polymorphisms, but the replications studies have been mixed and insufficient as of 2005 for a meta-analysis. Studies also indicate an association of BDNF to suicidal behavior. However, findings from the gene-environment interactions studies suggest that the current BDNF models of depression are too simplistic. A 2008 study found interactions (biological epistasis) in the signaling pathways of the BDNF and the serotonin transporter; the BDNF Val66Met allele, which was predicted to have reduced responsitivity to serotonin, was found to exercise protective effects in individuals with the short 5-HTTLPR allele that is otherwise believed to predispose individuals to depressive episodes after stressful events. Thus, the BDNF-mediated signalling involved in neuroplastic responses to stress and antidepressants is influenced by other genetic and environmental modifiers.
Fructose malabsorbtion
A series of 50 subjects examined in an Australian study showed increased depressive scores on Beck Depression Inventory associated with reduced plasma tryptophan, due to Fructose malabsorption
